Phytophthora kernoviae
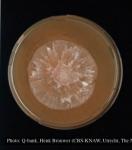
Phytophthora kernoviae Brasier, Beales & S.A. Kirk (2005) was first observed in Cornwall, southwest England in 2003. The new species was described in 2005. It causes leaf lesions on rhododendron and stem lesions on European beech in gardens and woodlands in the UK and Ireland. Other trees and shrubs are also affected. The pathogen appears to match isolates recovered in New Zealand from a diseased cherimoya orchard in 2006 and soil isolates recovered previously from a native kauri forest and a radiata pine plantation. P. kernoviae has also been isolated from fallen leaves of Drimys winteri and streams in a native South American forest. Etymology: ‘Kernow’, the old name for Cornwall.
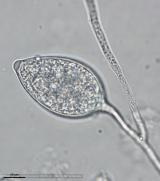
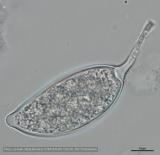
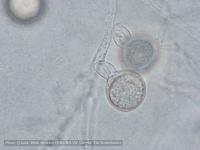
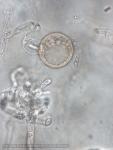
Sporangia (34-52 x 19-31 µm, mean range ca 38.5-45.5 x 22.3 x 27 µm) papillate and caducous, formed occasionally on carrot agar (CA) in the light, produced abundantly on CA plugs in nonsterile pond water or soil leachate, ovoid, limoniform to asymmetric or ‘mouse-shaped’, most with a conspicuous vacuole, pedicel length 5-19 µm, borne on sympodially branched sporangiophores. Hyphae sometimes denticulate or tuberculate. Chlamydospores not observed. Colonies in dark on CA largely submerged with small central area of aerial mycelium, with alternating rings of mycelium in diurnal light. Homothallic, gametangia abundant on CA after 10 d. Oogonia diameter 21-28 µm (mean 23.5-25.5 µm), often with tapered stalks. Oospores 19-25 µm (mean 21.1-22.5 µm), plerotic, wall thickness 3.5-5 µm (mean ca. 3.5 µm). Antheridia amphigynous, commonly 10-14 x 9-12 µm. Compared to UK isolates described above (Brasier et al., 2005), New Zealand isolates are reported to grow somewhat slower at 20° C and have a few small differences in the size of oogonia, sporangia, and pedicel length (Ramsfield et al. 2009).
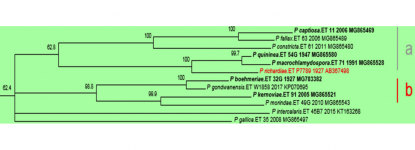
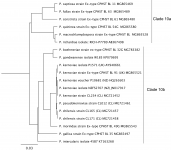
P. kernoviae is placed in Clade 10b (Blair et al., 2008), along with P. pseudokernoviae nom. prov., P. chilensis nom. prov., P. morindae, P. boehmeriae, P. gondwanensis, P. gallica, and P. intercalaris. P. pseudokernoviae nom. prov. and P. chilensis nom. prov. are claimed to be the closest relatives of P. kernoviae based on a multigene (ITS, LSU, ß-tubulin, HSP90, EF-1α, cox1 and NADH1) phylogenetic analysis (Jung et al. 2019). A possible origin in the southern hemisphere is hypothesized for P. kernoviae, and although present in New Zealand since at least 1953, it is not known if it is an endemic species (Ramsfield et al. 2009). Whole genome sequencing of UK, New Zealand, and Chilean isolates indicates greater genetic variation in the New Zealand isolates, lending support to a New Zealand origin for this species (Studholme et al. 2019).
Clade 10 phylogenetic tree from http://idtools.org/id/phytophthora (left).
ITS tree prepared 10.21.2019 using clade 10 accessions reported by IDphy database and Jung et al. 2018 (right).
Temperature optimum ca. 18° C, max. ca. 26°. Growth rate in the dark at 20° C on carrot agar 3.8-4.6 mm/d (mean 4.2 mm/day). Compared to UK isolates (Brasier et al., 2005), New Zealand isolates are reported to grow slower (2.7-4.0 mm/d) in the dark at 20° C on carrot agar. Growth rate of Chilean isolates is unknown.
P. kernoviae may be distinguished from other homothallic species with caducous, papillate sporangia with medium-length pedicels by its lower optimal temperature (cfr. P. botryosa and P. hevea); higher optimum temperature (cfr. P. nemorosa), often tapered oogonial stalks (cfr. P. meadii, P. botryosa, P. nemorosa), often asymmetric sporangia (cfr. P. meadii, P. megakarya, P. nemorosa), and longer pedicels (cfr. P. boehmeriae). P. kernoviae is purported to be distinguishable from P. pseudokernoviae nom. prov. and P. chilensis nom. prov. based on pedicel length, complexity of sporangial sympodia and oogonia characteristics (Jung et al. 2019). Compared to P. ramorum, which often occurs in similar habitats in the UK, P. kernoviae is homothallic instead of heterothallic, is papillate instead of semi-papillate, does not produce chlamydospores, and has a longer pedicel length.
The searchable web-based databases http://phytophthora-id.org/ and http://www.idtools.org/id/phytophthora/are useful for rapid identification of Phytophthora species based on sequencing of the ITS or Cox spacer regions, followed by BLAST searching the database. These databases only include sequences that are associated with published Phytophthora species descriptions, classic Phytophthora phylogenetics references, and type or well-authenticated specimens.
For more information about Phytophthora kernoviae, visit our Disease, Education and Management materials, and Reference sections.